Winter Salt
Winter road salt, which contains chloride, has become an environmental issue of great concern in this watershed and beyond. Winter salt, which can include sodium, calcium and magnesium chloride is the substance we apply on roads, sidewalks and parking lots to keep them clear of ice and make them safer for travelling.
What's the problem?
In short, we’re applying too much, chloride concentrations are steadily increasing, and it’s having negative environmental consequences.
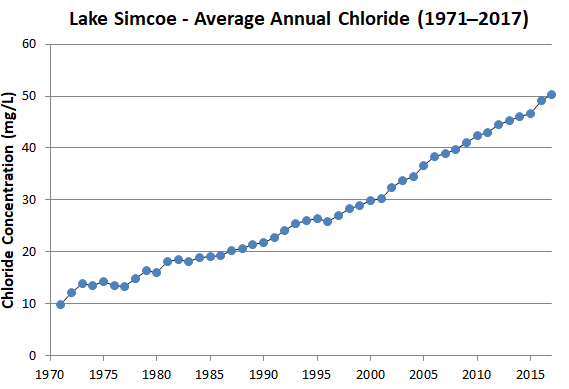
Our monitoring activities have identified a disturbing increase in salt in our rivers and streams and Lake Simcoe itself (see graph).
The problem with chloride is that it’s highly soluble. This means that once it dissolves into the water, there’s really no effective way at getting it out. And our plants and animals are accustomed to fresh water. So the increasing salt concentrations are hurting them.
So we need to address the issue by trying to reduce the amount of salt we use since it’s really our only option. If we don’t, salt is poised to overtake phosphorus as the biggest issue in the watershed.
A look at the numbers
To understand the extent of the problem, let’s take a look at some numbers around chloride (salt) concentrations in the water.
The Canadian government sets out water quality guidelines that define the levels at which chloride effects aquatic life. There are two different guidelines: a level for long-term exposure and another for
short-term exposure.
Long-term exposure is a maximum of 120 milligrams of chloride per litre of water (120 mg/L). The exposure limit for short-term exposure is 640 milligrams of chloride per litre of water (640 mg/L). Severe effects to aquatic life can be expected in as
little as 24 hours when the acute exposure is exceeded.
In our streams and rivers, particularly in urban areas, we are seeing regular exceedances of the short-term and even long-term guidelines. For example, a concentration of 10,000 mg/L was found in Western Creek (in Newmarket) in January and February 2018.

Not enough people realize how bad it is
Salt tastes great on your fries… so how bad can it be? Perhaps the reason people don’t associate road salt with an environmental problem is exactly because we put the substance on our food.
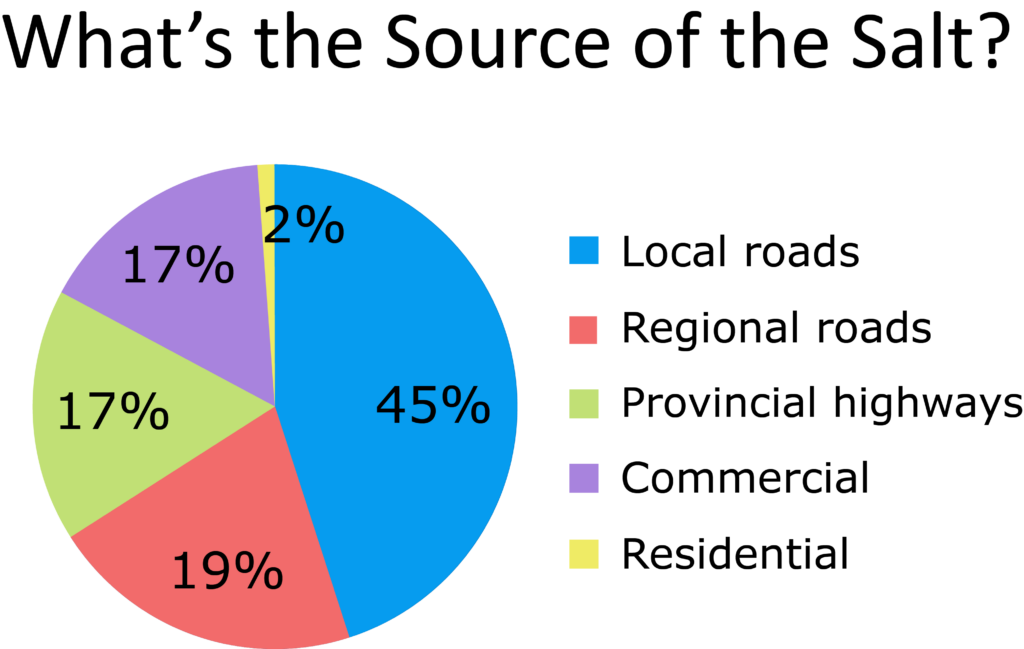
The biggest problems are roads and commercial parking lots
Making a dent in the amount of winter salt we use means addressing roads and parking lots, the largest contributors of winter salt.
Maybe it's all in our heads?
What We're Doing
Continued monitoring – We continue to undertake regular monitoring of chloride concentrations throughout the watershed.
Partnering for change – We think the answers depend on collaboration. We’ve created and participate in salt working groups, bringing together experts from the public sector, private sector, non-governmental organizations, legal community, regulators and academics.
Research to inform guidelines – This winter our researchers will be using a friction tester to track the results of salt application on various surfaces in the watershed. The goal is to determine the right amount of salt to achieve safe conditions. Our friction tester uses the same technology as airport testers.
Parking lot design with winter in mind – We’ve developed parking lot design guidelines. If these guidelines are followed, less winter salt would be required. We’re also encouraging municipalities to incorporate these guidelines into their policies.
What You Can Do
You are key to the solution! What you can do for the environment when it comes to winter salt use is best summed up with three P’s: Preparation, Patience and Pressure.
Prepare: Shovel your driveway and sidewalk as soon as possible so ice doesn’t have a chance to form. Wear sturdy winter boots. Use snow tires. Redirect downspouts away from paved areas so ice can’t form.
Patience: When it snows it often takes longer to get places. Consider leaving early, or accept that you may be late. This is winter in Canada!
Promote: If you see excessive salt being applied, speak to the property owner. Tell them about the environmental consequences of applying too much. Ask them to contact us. We can direct them to agencies like Smart About Salt® to have their staff or contractors trained in correct winter salt use.
Check out this video from the U.S. Consulate General Toronto about salt!
Here’s the basics on how salt works
Salt lowers water’s freezing point, the temperature at which it changes from a liquid to a solid and vice versa.
How quickly salt melts frozen water depends on a number of variables, including temperature, time, and the rate of application.
Fortunately, it is usually not necessary to melt all the snow and ice. Merely destroying or preventing the bond between pavement and frozen water is a more efficient, economical and environmentally sensitive approach. Once the bond is broken between the pavement and the ice, it’s easier for plows to scrape the ice off the surface.
Technically, any substance that dissolves in water lowers its freezing point. Sugar can lower water’s freezing temperature, but salt’s lower molecular weight makes it six times more effective.
Interesting facts
Chloride Numbers at a glance
- 10 mg/L or less: typical background chloride concentrations found in natural surface water
- 120 mg/L: long-term exposure
- 640 mg/L: short-term exposure
- 49 mg/L: average chloride concentration in Lake Simcoe
- 6,120 mg/L: highest level recorded in our watershed (Hotchkiss Creek, Barrie, February 2013)
- 19,250 mg/L: chloride concentration in seawater
Listen to CBC’s Metro Morning:
Why the U.S. Consul General in Toronto is raising the alarm about road and sidewalk salt
![]() Get Smart About Salt
Get Smart About Salt
Smart About Salt® is a voluntary not-for-profit organization that offers training to improve winter salting practices and recognizes industry leaders through certification.
![]() Parking Lot Design
Parking Lot Design
When parking lots are designed with winter maintenance in mind, they naturally require less salt application to maintain the same level of service without increasing liability.
![]() Related Documents
Related Documents
Sand versus Salt: Should sand be used for winter maintenance?